- Home
- News
- Spotlight on Science
- X-ray tomography...
X-ray tomography reveals the contribution of different mechanisms to the instability of iron-based fuel cell catalysts
18-07-2023
There are a number of processes that can alter the stability of iron-based fuel cell catalysts, but it is yet unclear how these mechanisms affect catalytic performance. In this work, different testing protocols were combined in order to decouple the contribution of each mechanism to overall instability. X-ray fluorescence computed tomography was used at beamline ID16A to quantify the iron lost by a catalyst during one of the protocols.
H2-powered proton exchange membrane fuel cells (PEMFCs) are well suited for zero-emission automotive applications, but their broad commercialisation is hindered by their excessive cost. The latter would be significantly reduced if the Pt-based materials currently used to catalyse the reduction of O2 in PEMFC cathodes could be substituted with inexpensive, iron (Fe-) based catalysts. However, these materials suffer from a rapid deterioration in performance that has been ascribed to the following mechanisms: (i) the dissolution (or demetallation) of the Fe in the active sites that catalyse the reduction of O2; (ii) the corrosion of the carbon matrix hosting those sites; and (iii) the damage to the active sites and carbonaceous matrix caused by radicals derived from hydrogen peroxide produced as an O2-reduction by-product. Most importantly, the relative contributions of these mechanisms to the overall instability endured by the catalyst during fuel cell operation are largely unknown.
Click image to enlarge
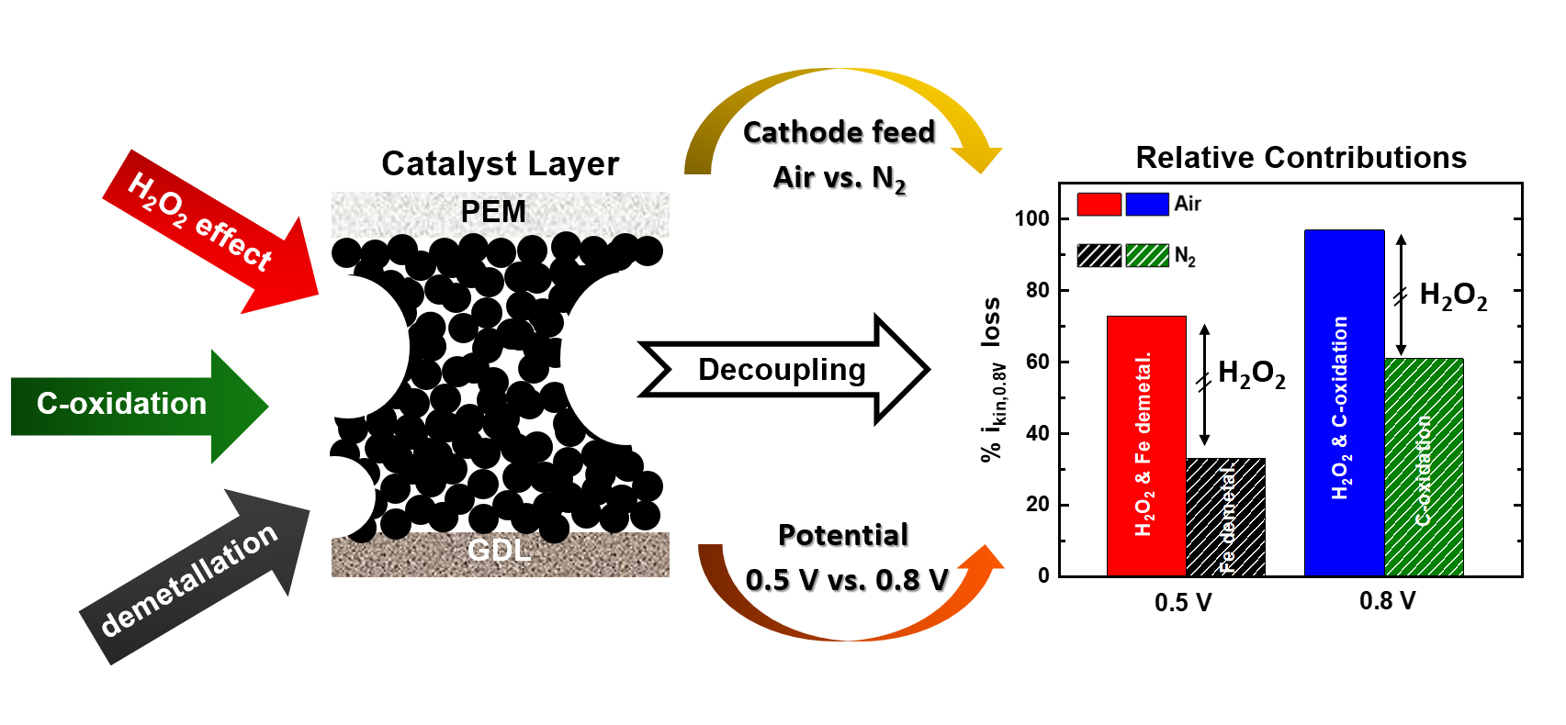
Fig. 1: From left to right: schematic representation of the three processes causing the instability of Fe-based catalyst layers (H2O2-related effects, electrochemical carbon oxidation and demetallation); their decoupling using a combination of gaseous cathode feeds (air vs. N2), potentials (0.5 vs. 0.8 V) and hold durations; and the relative contributions to the overall kinetic current losses at 0.8 V (ikin,0.8V) estimated through these protocols.
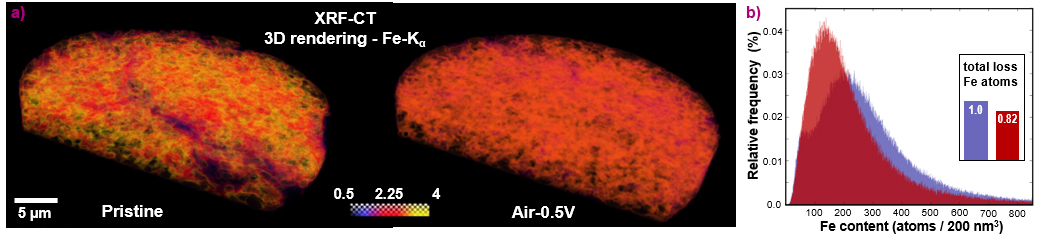
To shed light on this issue, four PEMFC-testing protocols were integrated in this work. These procedures involved different potential hold values and durations, along with the use of air vs. an inert gas (N2) as the PEMFC cathode gas feed. This approach allowed to quantify for the first time the relative influence of the above mechanisms in the overall performance decay undergone by an Fe-based catalyst, as illustrated in Figure 1. Complementarily, 2D X-ray fluorescence (XRF) mapping and 3D XRF computer tomography (XRF-CT) was used at beamline ID16A to characterise as-prepared and post mortem electrodes of the same catalyst (Figure 2a), and determined that ~ 15 % of its initial Fe-content dissolved during one of the PEMFC stability protocols (Figure 2b). This finding differed from energy dispersive X-ray spectroscopy (EDS) investigations, which did not detect statistically significant Fe losses. This was made possible by the superior sensitivity and about 200-fold larger (and thus statistically more meaningful) sample volume probed by XRF-CT compared to EDS.
Click image to enlarge
Fig. 2: a) 3D rendering of the Fe Kα line for the pristine (left) and post mortem (right) Fe-based catalyst layers based on tomographic reconstructions of the XRF-CT measurements taken at beamline ID16A. The colour scale is limited between 0 and 4 ng·mm–2. b) Fe distributions within the pristine (purple) and post mortem (red) catalyst layers inferred from 3D XRF-CT, with the inset depicting the total loss of Fe atoms throughout the complete volume of 6000 μm3.
In summary, this work describes a novel protocol allowing to decouple the contributions of different mechanisms to the instability of Fe-based O2-reduction catalysts, while providing precious information on their demetallation through XRF-CT measurements. These findings could help to design non-noble metal catalysts with the sufficiently high durability required for their implementation in commercial PEMFCs.
Principal publication and authors
Decoupling the Contributions of Different Instability Mechanisms to the PEMFC Performance Decay of Non-noble Metal O2‑Reduction Catalysts, S. Ünsal (a), R. Girod (b), C. Appel (a), D. Karpov (c), M. Mermoux (d), F. Maillard (d), V.A. Saveleva (c), V. Tileli (b), T.J. Schmidt (a), J. Herranz (a), J. Am. Chem. Soc. 145, 7845 (2023); https://doi.org/10.1021/jacs.2c12751
(a) Paul Scherrer Institut (PSI), Villigen (Switzerland)
(b) École Polytechnique Fédérale de Lausanne, Lausanne (Switzerland)
(c) ESRF
(d) Université Grenoble Alpes − CNRS, Grenoble (France)
| About the beamline: ID16A |
| The ID16A Nano-Imaging beamline is designed for quantitative 3D characterisation at the nanoscale of the morphology and the elemental composition of specimens in their native state. It addresses research questions in metallo-biology, neurosciences, biomineralisation and advanced materials characterisation. The beamline provides a high-brilliance beam focused down to a few tens of nanometres to perform coherent imaging techniques, X-ray fluorescence microscopy and nanotomography. All measurements can be performed under cryogenic conditions to preserve the biological samples close to their native hydrated state and reduce radiation damage. |