- Home
- News
- General News
- Unlocking secrets...
Unlocking secrets of a key enzyme in magic mushrooms
12-04-2024
Scientists have found the process behind the biosynthesis of psilocybin – the natural hallucinogen found in magic mushrooms. The discovery may lead to tailor-made variants that could be used in treating mental health conditions like severe depression. The results are published in Nature Communications.
Depression is a widespread mental health condition that affects 264 million people worldwide, according to the World Health Organization. The hallucinogen psilocybin, the active substance in ‘magic mushrooms’, is being investigated as a potential therapeutic for depression cases where conventional treatment doesn’t provide relief, for substance dependence and for several anxiety-related conditions.
Recent clinical trials have shown promising results where a single dose of psilocybin has provided lasting remission for people who have otherwise struggled to heal. It has already received breakthrough therapy status from the US Food and Drug Administration and is expected to enter phase III clinical trials shortly. The journal Science designated the use of psychedelics for treating mental health conditions as one of the biggest breakthroughs of the year 2021.
The growing interest in potential medical uses of psilocybin has prompted efforts to biotechnologically manufacture the compound and investigate the therapeutic properties of new analogs.
Now scientists led by the Medical University of Innsbruck (Austria) have elucidated the structure of a crucial enzyme in psilocybin biosynthesis at various stages of the reaction cycle, with the help of the ESRF’s structural biology beamlines.
 |
|
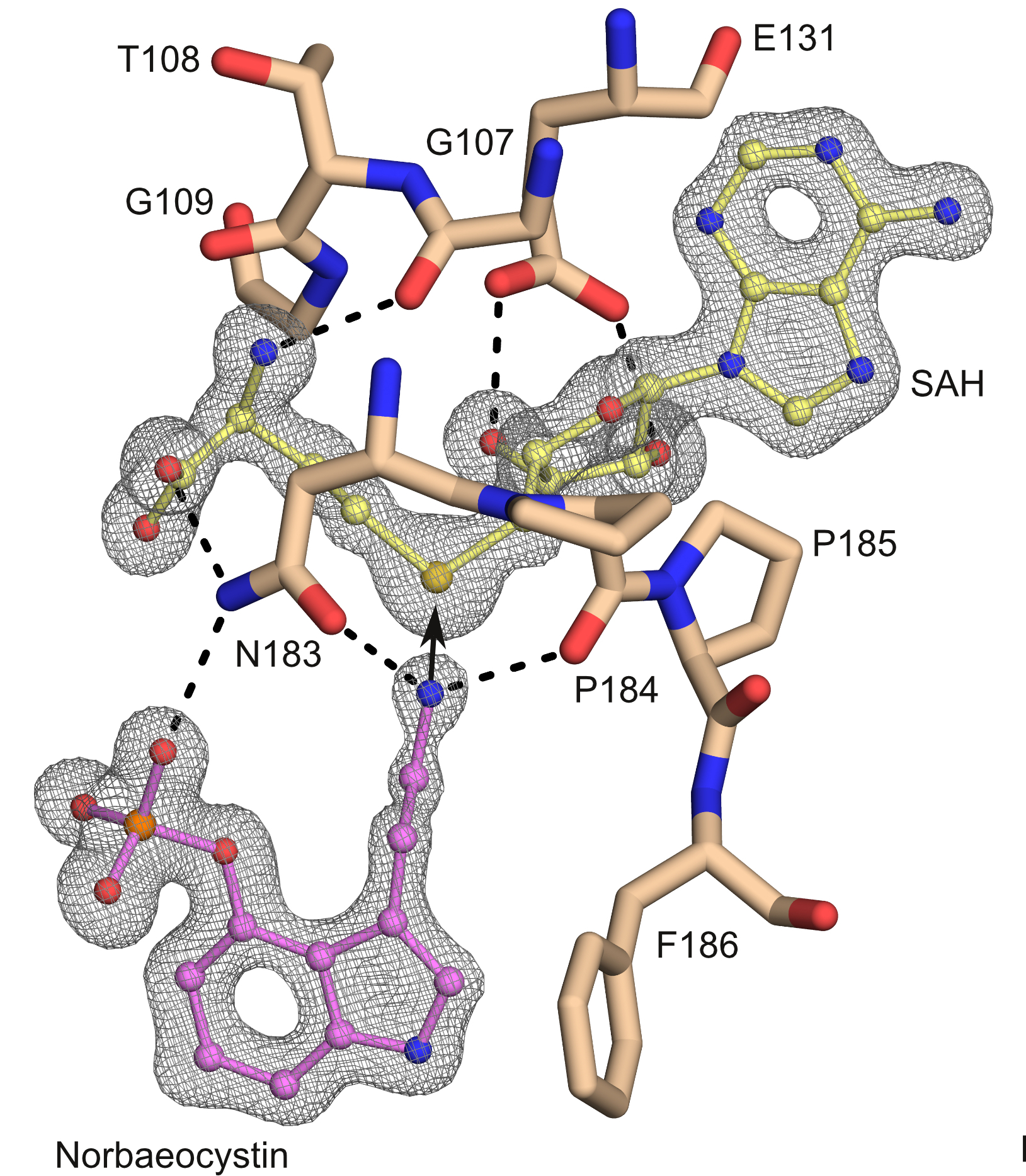
Electron density for norbaeocystin (the molecule at the bottom and to the left) and SAH (at the top). Also shown are important parts of the active site of the PsiM protein in beige. Dashed lines are hydrogen bonds. The arrow is where the so-called "nucleophilic attack" (the chemical reaction) would happen. Credits: Hudspeth, J., et al. Methyl transfer in psilocybin biosynthesis. Nat Commun 15, 2709 (2024) |
“Using the powerful ID23-1 beamline at ESRF helped us to obtain the best possible data and reach the exceptional final resolution of 0.9 Å. This is an astonishing level of detail that enables you to observe individual atoms within the enzyme” explains Sebastiaan Werten, structural biologist at the Medical University of Innsbruck and corresponding author of the study.
Delving into PsiM’s structure
The last step in creating psilocybin involves adding two methyl groups (dimethylation) to a specific molecule called norbaeocystin. The enzyme PsiM is responsible for this step. The team focused on understanding the structure and function of this enzyme.
By crystallizing PsiM in a series of complexes either containing the initial substrate norbaeocystin, the singly methylated intermediate baeocystin or the final product psilocybin, together with a coenzyme analog such as S-adenosyl-l-homocysteine (SAH), researchers have gained unprecedented insight into the three-dimensional structure of the enzyme and the consecutive steps in the biosynthetic process.
PsiM is incredibly specific in recognizing its target molecule, norbaeocystin. It wraps around the molecule tightly, guiding it through a series of precise chemical reactions. The researchers found that PsiM can adopt different shapes while interacting with its partners, which might be important for how it attaches onto the molecules initially and then processes them.
Evolutionary tinkering
The study also sheds light on the evolution of enzymes. PsiM belongs to a family of enzymes called METTL16, which were originally involved in gene regulation. However, over time, PsiM evolved to become a specialist in making psilocybin.
This evolution was likely driven by the similarities between psilocybin precursors and the RNA molecules that METTL16 enzymes were originally designed to work on. This means that PsiM still has some limitations inherited from its RNA-modifying ancestors. “What happened here is a beautiful example of evolutionary tinkering, where nature reuses an existing protein scaffold for a completely different function”, explains Werten.
These results could pave the way for bioengineering efforts to produce novel analogues of psilocybin with enhanced therapeutic properties. “Thanks to our new understanding of the intricate molecular mechanisms of psilocybin biosynthesis, we may now be able to tailor the chemical structure of the compound with the goal of improving its efficacy and safety for medical use”, concludes Werten.
Reference:
Text by Montserrat Capellas Espuny
Top image: Magic mushrooms. Credits: Adobe Stock



