- Home
- News
- General News
- #weekendusers How...
#weekendusers How do iron minerals behave in marine ecosystems altered by humans?
23-09-2016
Every day, human activity affects marine ecosystems by discharging waters from urban and agricultural areas. Dutch users investigate what role iron minerals play in the health of these ecosystems, using the X-rays of the Dutch-Belgian beamline (DUBBLE).
Humans pump essential elements (nutrients) such as phosphorus into the sea through detergents, sewage and fertilizers. These nutrients change the ecosystem and eventually the minerals that can form in the seafloor. Algae thrive in an environment with a lot of nutrients, which can lead to dense growths of (toxic) algae. When the algae die and sink to the bottom, bacteria use up all the oxygen in the water when they eat the dead algae. This creates dead zones without oxygen and without life other than bacteria; fish, crabs, bivalves and others leave or die.
|
Peter Kraal in the field. |
 |
|
Peter Kraal at DUBBLE, preparing samples. |
As disturbing as all this sounds, nature also provides solutions: iron in the seabed is very effective at removing phosphorus. But there is a hindrance, as Peter Kraal, researcher at Utrecht University (UU), explains: “Human activity can change ecosystems in such a way that iron minerals become less efficient at removing phosphorus from the water”.
So Kraal is at the ESRF this weekend, together with Case van Genuchten, a UU researcher and expert in drinking water treatment, and Simon Müller, a master student at UU. They experiment with various iron phases: “We go to the lab and make different types of iron minerals. Then we stick them in sediments in an estuary with an oxygen-depleted seabed in the Netherlands, and two weeks later we look at how they have transformed”, explains Kraal. The ESRF allows them to get an accurate picture of the structure of the iron minerals by using X-ray absorption spectroscopy (XAS) on the Dutch-Belgian beamline (BM26A).
 |
|
|
The Dutch estuary where the samples are placed. |
The samples in the estuary. |
The samples need to be kept in an environment without oxygen, so the team manipulates them in a plastic bag filled with nitrogen gas instead of normal air. In the bag, they make small pellets that are sealed and then brought to the beamline. For this experimental session, they have brought a total of 96 samples from eight different types of iron minerals that have been in the field.
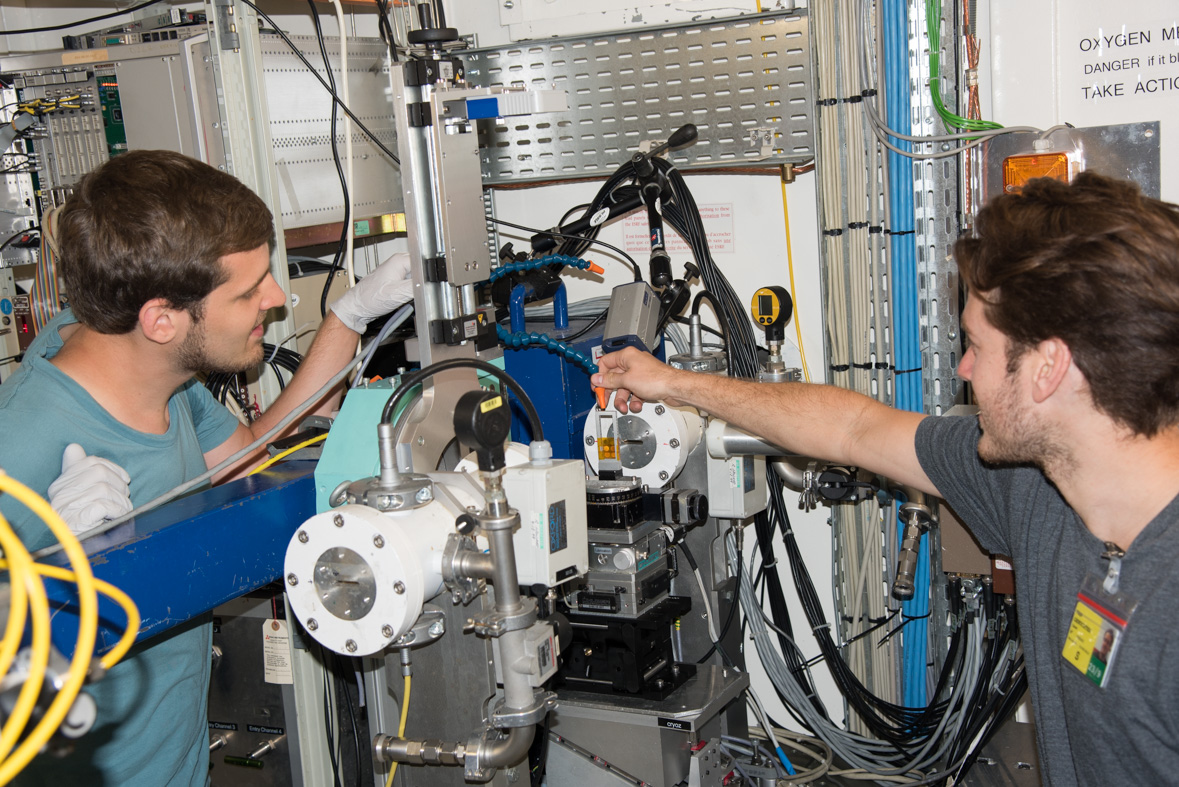 |
|
Case van Genuchten (right) and Simon Müller (left) putting a sample in the Experimental hutch. |
 |
|
The samples as powder. |
Text by Montserrat Capellas Espuny
Top image: Algal bloom off the coast of Denmark.



