- Home
- News
- Spotlight on Science
- A snapshot of bacterial...
A snapshot of bacterial signalling
06-04-2010
Bacteria sense external events and send signals to the cell interior via two-component systems formed by a membrane protein, histidine kinase, that has extracellular and intracytoplasmic domains and a soluble intracellular response regulator. The crystal structure of such a system from Thermotoga maritima provides the paradigm for a structural understanding of signalling in bacteria and for its perturbation using structure-based inhibitors. An additional highlight of this study is the demonstration that the histidine kinase component can autophosphorylate in cis geometry, refuting the belief that trans autophosphorylation is universal for histidine kinases.
An adaptive response to environmental stimuli is essential for life. The most widespread response mechanism involves the transfer of a phosphoryl group amongst the proteins in a signalling process. Two-component signal transduction systems are the chief signalling devices in bacteria and archaea. Actually, they are found in all life domains, although not in animals. A single bacteria can contain tens to hundreds of two-component systems controlling vital processes such as metabolism, development, motility, response to stress or virulence. These systems offer enormous possibilities for the development of new antimicrobials because they play a paramount role in bacterial physiology and in virulence processes, allowing adaptation of a parasite to its human host, or even triggering resistance to known antibiotics.
Although there are numerous variations in the details, two components systems obey the same basic pattern. The prototype consists of two proteins. One of them is a homodimeric membrane sensor called histidine kinase which has one histidine residue in its intracellular part that is autophosphorylated in response to the stimulus (which is sensed by the histidine kinase extracellular domain). The other protein, called the response regulator, is in the cytoplasm, and interacts with the histidine kinase, accepting the phosphate and flying to the chromosome to influence the expression of certain genes. In some cases the targets are not genes but other proteins. The signal decays with dephosphorylation of the response regulator, which is accelerated by interaction with the cognate histidine kinase.
Despite the similarity of the different pairs of two-component systems, there is exquisite partner specificity within each pair, so that there is generally no cross-signalling between different couples. Until now, the structural basis for this selectivity remained a mystery. Since the two components have to associate for both phosphorylation and dephosphorylation of the response regulator but the conditions triggering one or the other activity differ, there should be different modes of mutual recognition associated with these two activities. Another question requiring structural clarification was how the signal triggers autophosphorylation of the histidine kinase. Furthermore, this process was believed to occur in trans, that is, one subunit of the dimer phosphorylating the other subunit, but the structural foundations for trans-autophosphorylation remained unclear. Finally, the chemical mechanisms of the three phosphoryl transfer processes catalysed by each system -autophosphorylation, phosphorylation and dephosphorylation of the response regulator- were mainly conjectural.
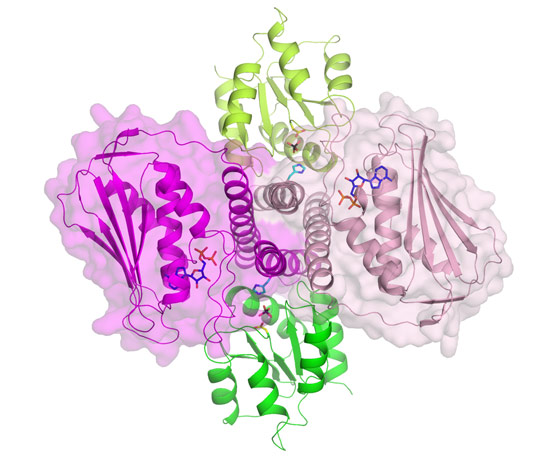
Spanish scientists from the Instituto de Biomedicina de Valencia-CSIC (Spain) have provided responses to most of these questions and have shattered the view that autophosphorylation of the histidine kinase component occurs exclusively in trans geometry. They have determined the crystal structure of the complex formed by the intracellular part of a histidine kinase of the bacteria Thermotoga maritima and its cognate response regulator, using beamlines BM16, BM30A and ID14-3. Figure 1 presents a view of the dimeric histidine kinase and response regulator complex. The structure mimics the histidine kinase-catalysed dephosphorylation of the response regulator, but it also sheds light on phosphotransfer to the response regulator. The structure suggests cis rather than trans-phosphorylation of the histidine kinase component, and, in fact, the group proves biochemically that this is actually the case, also proving cis-autophosphorylation in another two-component system from Staphylococcus aureus. The structure allows a mechanism to be proposed for histidine kinase mediated-signalling from the cell exterior to the intracellular part of the histidine kinase. The detail provided by this complex, and the mechanistic and specificity-clarifying insight obtained, offers hopes for practical developments based on this structure.
Principal publication and authors
P. Casino (a,b), V. Rubio (a,b) and A. Marina (a,b), Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction, Cell 139, 325-336 (2009).
(a) Instituto de Biomedicina de Valencia-Consejo Superior de Investigaciones Científicas (IBV-CSIC), Valencia (Spain)
(b) Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER) (Spain)
Top image: Detailed view of the active site of the bacterial signalling complex to highlight the involvement of the protein components; histidine kinase in pink and its response regulator in green.